Plumbing and Mechanical --- Radiant & Hydronics Report 2016

Water Quality Matters
Demineralized water is preferred in hydronic systems.
Water is the lifeblood of hydronic systems. Its chemical characteristics can make the difference between a system that lasts for decades vs. one that develops expensive corrosion issues within months of commissioning.
So, just as we strive to maintain the health of our own circulatory systems, it’s important to maintain the health of water and water-based solutions that circulate through the hydronic systems we create and service.
Pure water consists of only hydrogen and oxygen. It is colorless, tasteless, and odorless. Unfortunately, “pure” water does not exist in nature. Because water is the universal solvent, water from springs, wells, and even municipal mains contains dissolved minerals such as calcium and magnesium, sediments such as fine silica sand or organic particles, and dissolved gases such as oxygen, carbon dioxide, and hydrogen sulfide. Some water sources also contain microorganisms such as bacteria or algae. There can be wide variations in these impurities from one water source to another.
In most hydronic systems, the unknown chemical characteristics of the water used to fill the system have the opportunity to interact with several materials and other chemicals already in the system. These include metals such as copper, brass, cast iron, stainless steel, and carbon steel, liquids such as glycol-based antifreeze and thread-cutting oils, and greasy residue such as solder flux. This complex combination of chemicals and metals has the potential to create undesirable reactions such as oxidation, sludge formation, galvanic corrosion, and pitting corrosion.
Unfortunately, the chemistry that could take place in newly crafted hydronic systems is often ignored. Many systems are just filled with whatever water is available on site.
Some systems will provide years of reliable service because of a “lucky” set of circumstances involving the purity of the water source, the materials used in the system, the care taken when assembling the system, and its subsequent operation and maintenance. Other systems will experience premature component failure due to an “unlucky” combination of these factors.
Lose the Ions
One of the best ways to minimize the potential for undesirable chemical reactions within hydronic systems is to demineralize that water. As its name implies, demineralization involves removing undesirable minerals, such as the dissolved calcium and magnesium compounds found in most groundwater and municipal water systems.
From a process standpoint, demineralization is similar to water softening. Both are called “ion exchange” processes. The undesirable ions in the source water — which come from dissolved salts containing calcium, magnesium, manganese, and chlorine — are captured and exchanged for ions that do not precipitate out of water when it’s heated.
In a typical water-softening process, the source water is passed through a column containing tens of thousands of small polymer resin beads that are chemically structured to attract undesirable ions such as those mentioned above. When an undesirable ion bond to a resin bead, another ion, specifically a sodium ion (Na+) is released from that bead. This give and take of ions is necessary to maintain the chemical charge balance of the water.
As the source water passes through a column filled with these resin beads, it will be largely stripped of the calcium and magnesium ions that can precipitate out of solution as the water is heated, and hardened into scale. The sodium ions that replace the original ions are more soluble in heated water and won’t form scale.
Softened water is certainly better than the original water from the standpoint of its ability to form scale, but it’s still not “pure water.”
The presence of sodium ions in the softened water leaves the water with relatively high electrical conductivity. This characteristic makes the likelihood of galvanic corrosion between dissimilar materials in the system (such as copper and iron) more likely. Softening solves one problem (e.g., removing calcium and magnesium ions from the source water, which would otherwise cause scaling), but leaves the water in a condition that could lead to other problems (e.g., galvanic corrosion).


Lose the Sodium
The preferred ion exchange process for water used in hydronic systems is demineralization. It also involves passing the source water through a column filled with resin beads. However, these beads are different than those used in water softeners. They are chemically structured so that when they capture an undesirable ion, they release either a hydrogen cation, which is chemically represented as (H+), or a hydroxide anion, which is chemically represented as (OH-). The hydrogen cation has one positive charge, and the hydroxide anion has one negative charge.
What do you think happens when one hydrogen cation (H+) meets up with one hydroxide anion (OH-)? Hint: Look at the number of hydrogen and oxygen atoms present, and remember that + and - charges attract.
That’s right! The cation and anion bond to form a neutral molecule of pure water (H2O).
Figure 1 illustrates the overall ion exchange process used for demineralization.
Water that has been demineralized to the point where its total dissolved solids reading is 10 to 30 parts per million has characteristics that are ideal for water-only systems, and as “base water” to be combined with inhibited glycol antifreeze or with additional chemical stabilizers.
A Simple Procedure
There are two ways to demineralize the water used in hydronic systems:
The first approach is good for smaller systems, where the flow rate of water through the demineralizer is sufficient to purge air from the system.
The second method is better in systems where the flow rate through the demineralizing column is not sufficient to purge air from the system.
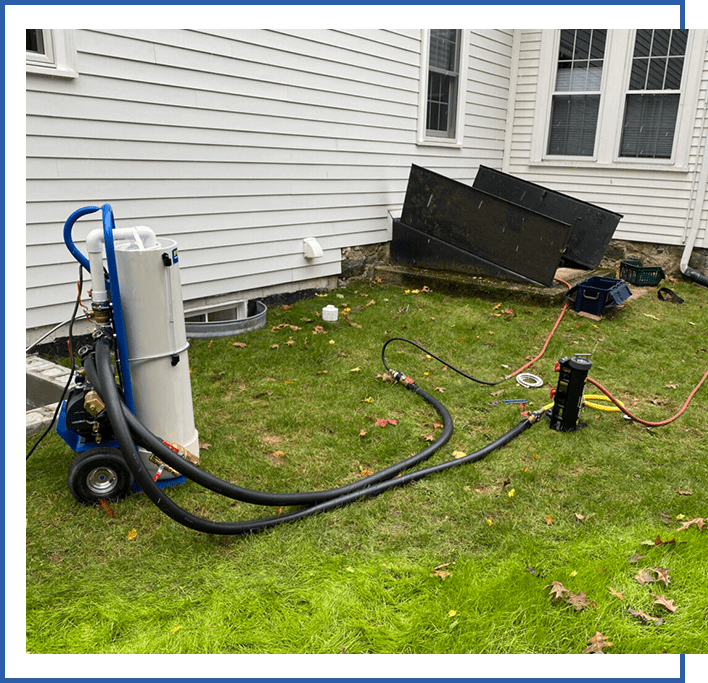
If using the second method, the demineralizing column is typically set up as a side arm device, as shown in Figure 2. The system piping is equipped with either a combination purging valve or three ball valves.
One hose brings water from the system to the demineralized, the other returns the processed water back to the system. The inline ball valve between the inlet and outlet valves is partially closed to create a pressure differential that drives some flow through the demineralizing column. The system’s circulator must be on to create this flow.
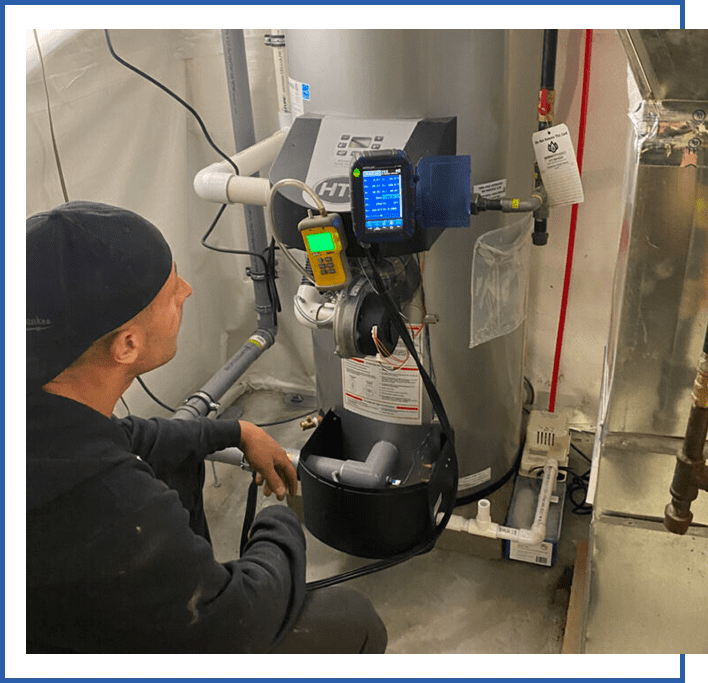
After the water has passed through the demineralizer for a few minutes, draw a sample of water from a drain valve and test it for a total dissolved solids value. The TDS reading can be found using a low-cost TDS meter, such as the one shown in Figure 3.
TDS is an inferred value based on the water’s electrical conductivity. Groundwater can have TDS values between 50 and 500 ppm. Seawater can have a TDS value as high as 40,000 ppm.
The water should be demineralized until it has a TDS value between 10 and 30 ppm. This is low enough to minimize any potential for scaling or galvanic corrosion, but still high enough to allow low-water cutoff devices (which rely on electrical conductivity) to operate. Once the TDS value of the water is reduced to this range, the demineralization process is complete. Close the valves, remove the hoses, and drain the remaining water from the demineralization column.
The Full Course
Although demineralization is the primary method for improving water quality in hydronic systems, I also recommend that you precede it with a thorough internal washing of the system, and follow it by adding a stabilizing chemical formulation.
Internal washing is done by filling the system with water, adding a “hydronic detergent” formulation, which is now available from several suppliers, and circulating this blend through the entire system, with the heat source operating at normal temperature, for at least one hour. The hydronic detergent breaks down oils and grease within the system and suspends them in the circulating fluid. At the end of the wash cycle, this fluid is completely drained from the system and disposed of based on the detergent supplier’s directions. Most of the currently available detergents are biodegradable and easy to dispose of.
After the water has been demineralized, I also recommend adding a film-forming hydronic system stabilizing formulation. Again, several such products are available in North America. They form extremely thin protective films on the inside of the piping components. They also contain chemicals to stabilize the pH of the fluid and help protect the system against the slow but unavoidable oxygen diffusion that occurs over the system’s life.
The icing on the demineralized water cake is to include high-performance air and dirt separation within the system. These functions can be achieved with separate devices or a single device. If the system contains any ferrous metals such as cast iron or steel — as most systems do — the dirt-separating device should include a magnet to capture ferrous oxide particles. I also recommend dirt separation for systems containing high-efficiency circulators with permanent magnet motors.
The health of the water in hydronic systems is important and has been largely overlooked by the North American hydronic industry for years. It’s time to correct for previous complacency and get serious about water quality in every hydronic system. The above procedures will guide you, and the results will reward you with good performance and long service life.
John Siegenthaler, P.E., is a consulting engineer and principal of Appropriate Designs in Holland Patent, N.Y. His latest textbook, “Heating With Renewable Energy,” was released in January from Cengage Publishing. It shows how to use modern hydronic technology to create systems supplied by solar thermal, heat pumps, and biomass heat sources. Additional information is available at hydronicpros.
